Why tracking
genotype-specific HPV persistence matters

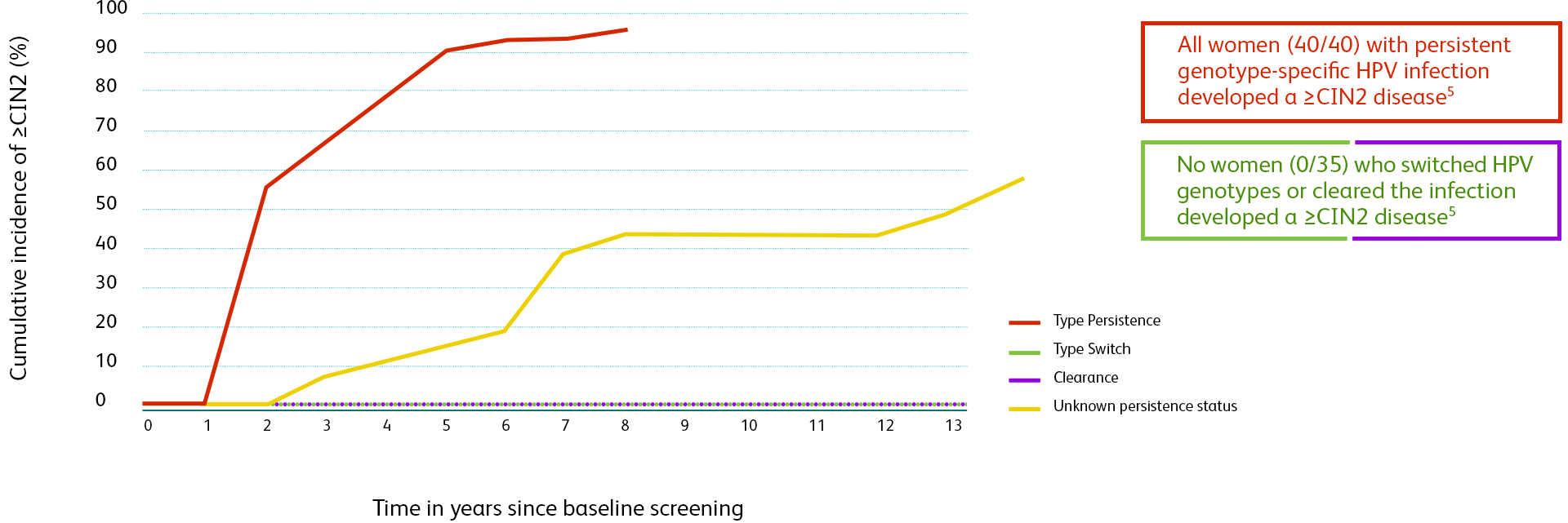
HPV infection history and current HPV results are both critical when assessing the risk for cervical cancer.1

Persistence of infections by high-risk HPV genotype is the single greatest risk factor for malignant progression.2