The clinical value
of extended genotyping

"Extended genotyping" refers to those high-risk HPV types beyond types 16 and 18 that may impact patients and lead to high cervical disease risk.1

Extended genotyping enables risk stratification1 and persistence monitoring2,3 to help prevent more cases of cervical cancer.
As the vaccinated population increases, the prevalence of high-risk HPV types beyond 16 and 18 may change.
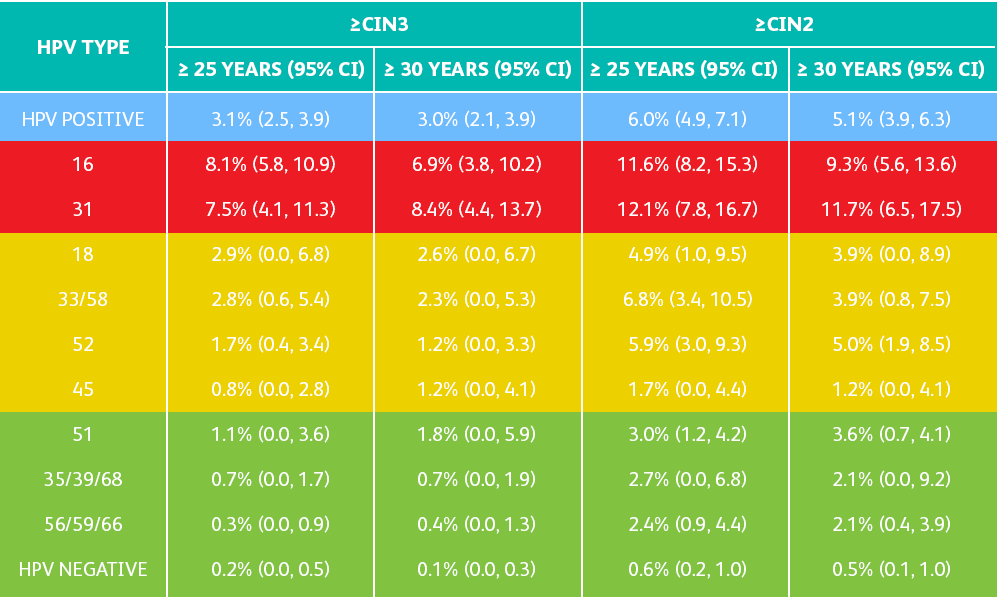
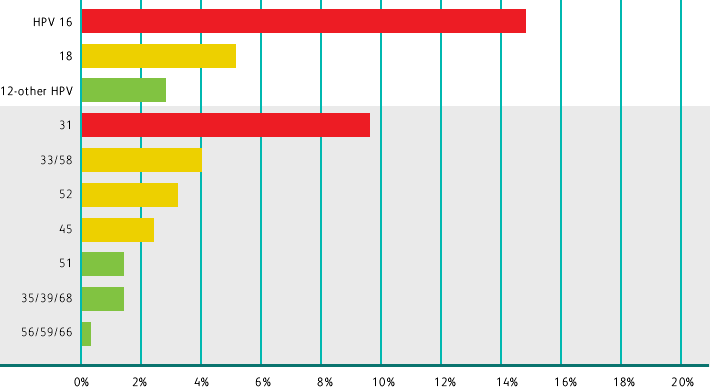
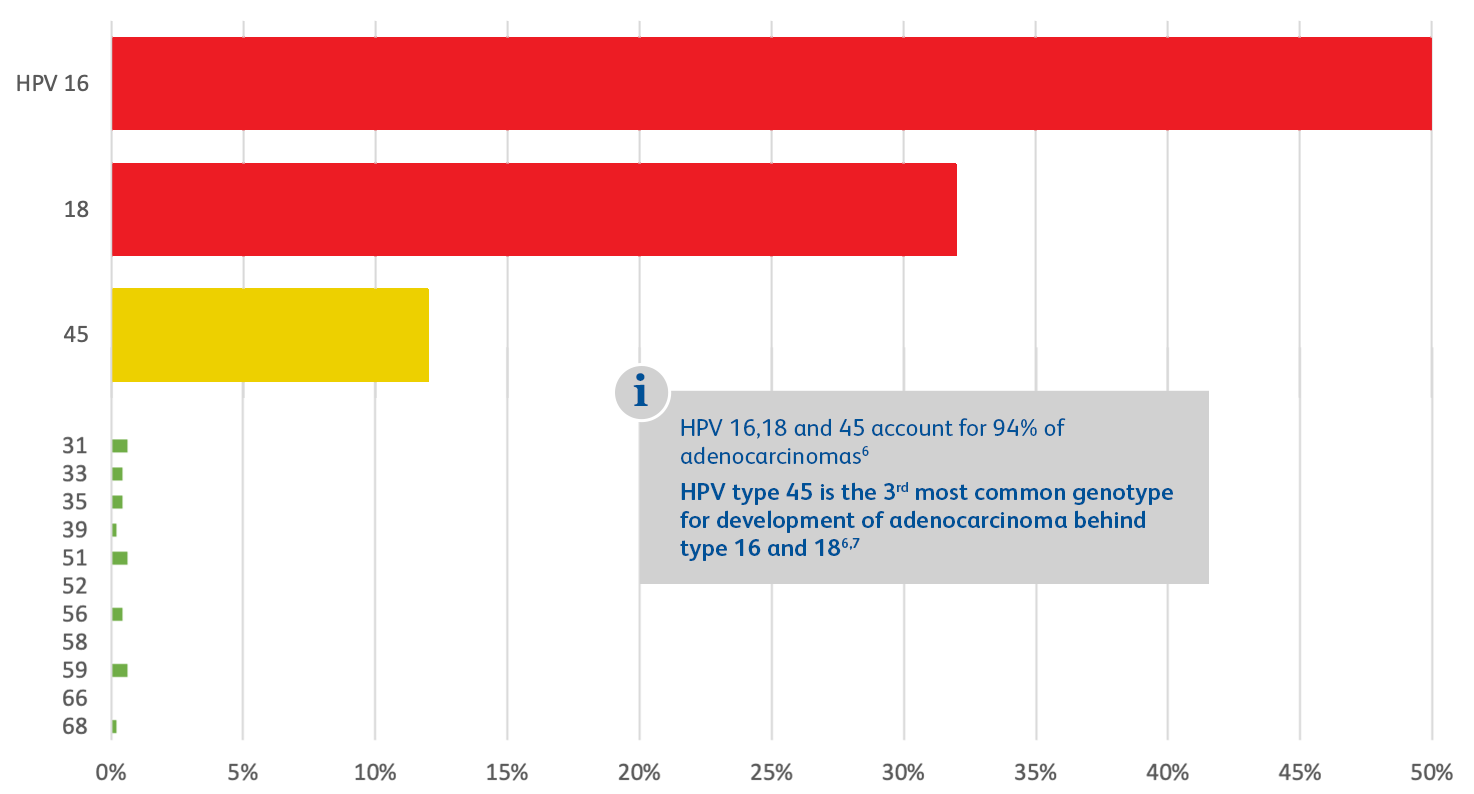
THE BD ONCLARITY™ HPV ASSAY PROVIDES VISIBILITY INTO HIGH-RISK GENOTYPES
BEYOND HPV 16 AND 18
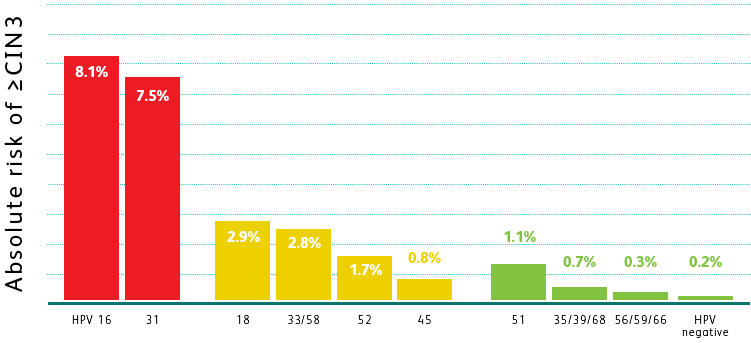

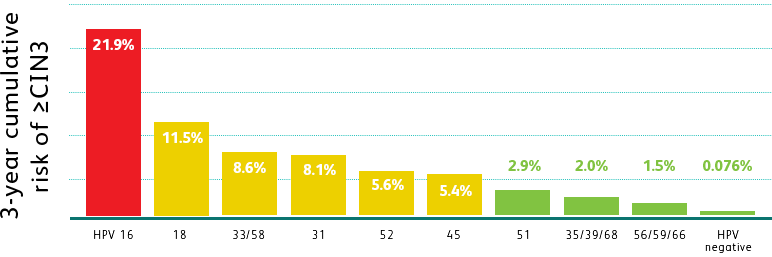 Adapted from Schiffman M et al. Int J Cancer. 2016;139(11):2606-2615.
Adapted from Schiffman M et al. Int J Cancer. 2016;139(11):2606-2615.