BD Onclarity™ HPV Assay
Extend the Power of HPV Testing

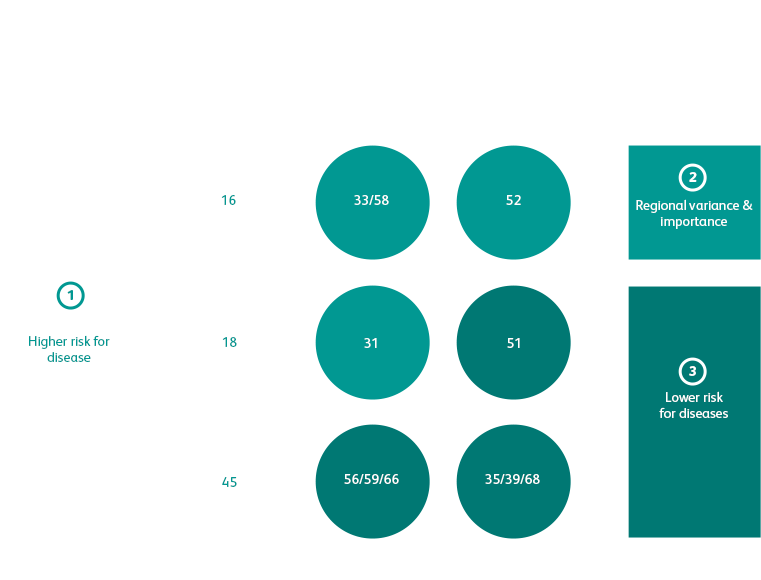
A 3 well, multiplexed design with genotype specific primers and probes1 (instead of consensus primers) provides excellent detection of single and mixed HPV infections.2

Simultaneous HPV detection and extended genotyping on-demand in 1 single run1 with no additional processing, reagents or labor.