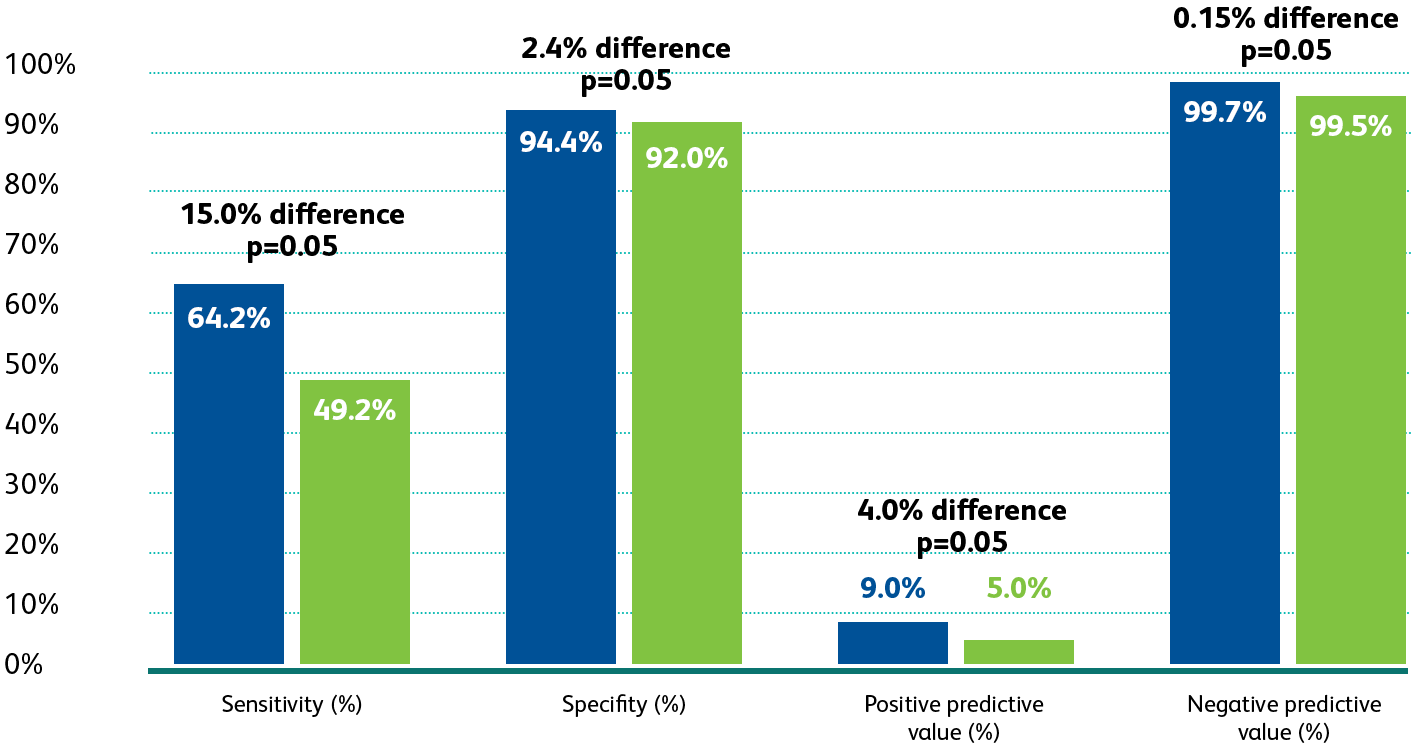
The BD Onclarity™
HPV Assay FDA Clinical Trial

The
BD Onclarity™ HPV Assay FDA Clinical Trial
1
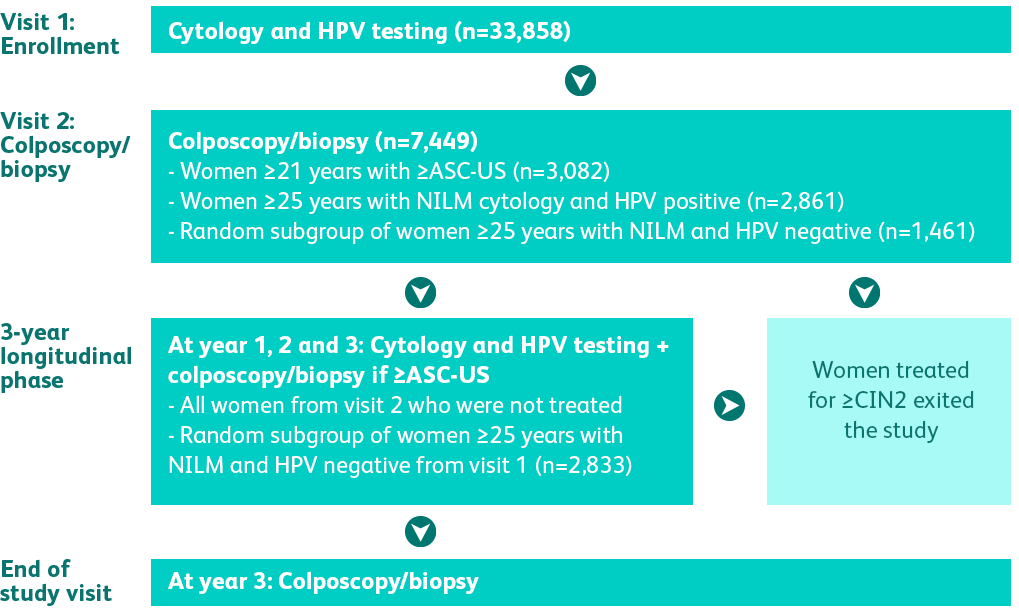
was a regulatory trial
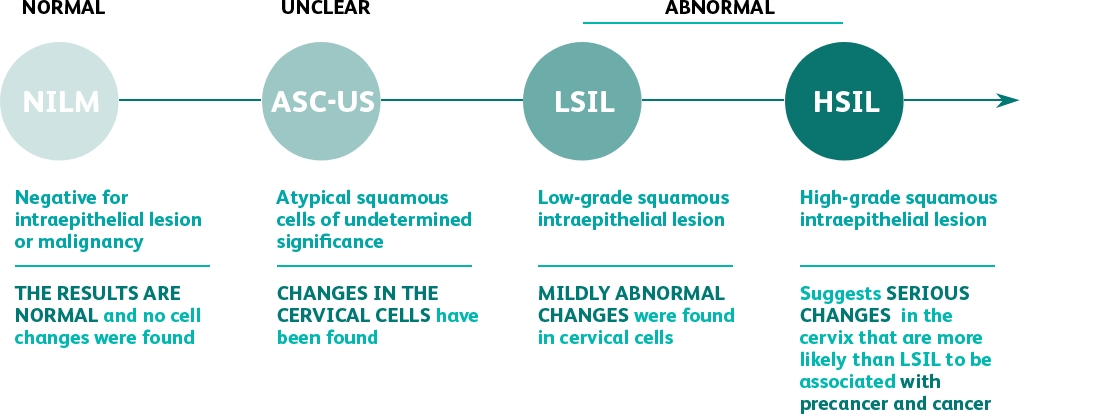
designed to obtain FDA-approval in the USA for high-risk HPV testing and genotyping for HPV 16, 18, 45 and beyond.

The first premarket approval trial to evaluate the
performance of extended genotyping beyond
HPV 16 and HPV 18
in the USA.
HPV 16 and HPV 18 in the USA.