Extended genotyping supports risk stratification and persistence monitoring to guide patient management1-5
- Genotypes 16 and 18 account for 70% of invasive cancer worldwide, but their prevalence is declining as vaccination rates increase6-9
- Genotypes 31/33/58 have a CIN3+ risk similar to genotype 18, but 51/35/39/68/56/59/66 have a much lower risk10,11
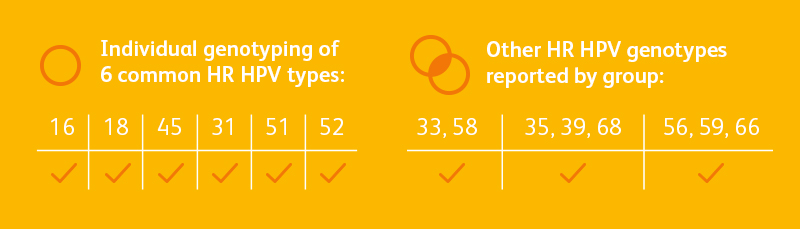
- The BD OnclarityTM HPV Assay reports individual results for 6 of the 14 high-risk genotypes and grouped results for the remaining 8 high-risk genotypes12
In the post-vaccination era the prevalence of high-risk genotypes may change making it crucial to identify high-risk genotypes individually
The BD OnclarityTM HPV Assay provides extended genotyping, offering the flexibility you need to adapt to changing clinical landscape and screening guidelines, including self-collection

HPV primary screening

Co-testing

Cytology primary + ASCUS reflex
Providing you with advanced accuracy, timely detection and actionable HPV genotyping so you can make more informed decisions
- The BD OnclarityTM HPV Assay is designed to minimize the risk of false-negative results by:
- Including an internal control
- Targeting the E6/E7 region of the HPV viral genome rather than the L1 region, which can be deleted during HPV DNA integration13
- The BD OnclarityTM HPV Assay is designed to minimize the risk of false-positive results by lacking cross-reactivity with low-risk HPV types12
- The BD OnclarityTM HPV Assay reports individual results for 6 of the 14 high-risk genotypes and grouped results for the remaining 8 high-risk genotypes12
- Make more informed decisions with the extended genotyping information you need to assess each patient’s risk for confident follow-up decisions 7-11
- New! The BD Onclarity™ HPV Assay supports vaginal specimens self- collected at either the clinic or in the home-setting12